 The three-dimensional (3D) spherically symmetric potential around atoms yields degeneracies known as shells, 1s, 2s, 3s, 3p,... Each shell can hold a specific number of electrons. The electronic configuration is particularly stable when these shells are completely filled wih electrons, occurring at 'magic' atomic numbers 2, 10, 18, 36,... In a similar way, the symmetry of a two-dimensional (2D), disk-shaped quantum dot leads to a shell structure with magic numbers 2, 6, 12, 20,... The lower degree of symmetry in 2D results in a different sequence of magic numbers than in 3D.
The three-dimensional (3D) spherically symmetric potential around atoms yields degeneracies known as shells, 1s, 2s, 3s, 3p,... Each shell can hold a specific number of electrons. The electronic configuration is particularly stable when these shells are completely filled wih electrons, occurring at 'magic' atomic numbers 2, 10, 18, 36,... In a similar way, the symmetry of a two-dimensional (2D), disk-shaped quantum dot leads to a shell structure with magic numbers 2, 6, 12, 20,... The lower degree of symmetry in 2D results in a different sequence of magic numbers than in 3D.By measuring electron transport through quantum dots, a periodic table of artificial 2D elements can be obtained. For this purpose, dots are connected via potential barriers to source and drain contacts. If the barriers are thick enough , the number of electrons on the dot, N, is a well defined integer. This number changes when electrons tunnel to and from the dot. However, due to Coulomb repulsion between electrons, the energy of a dot containing N+1 electrons is larger than when it contains N electrons. Extra energy is therefore needed to add an electron to the dot. Consequently, no current can flow which is known as the Coulomb blockade.
The blockade can be lifted by means of a third electrode closeby, known as the gate contact. A negative voltage applied to this gate is used to supply the extra energy and thereby change the number of free electrons on the dot. This makes it possible to record the current flow between source and drain as the number of electrons on the dot, and hence its energy, is varied. The Coulomb blockade leads to a series of sharp peaks in the measured current (see figure below). At any given peak, the number of electrons on the dot alternates between N and N+ 1. Between the peaks, the current is zero and N remains constant. The distance between consecutive peaks is proportional to the so-called addition energy, which is the difference in energy between dots with N+1 and N electrons. The magic numbers can be identified because significantly higher voltages are needed to add the 2nd, 6th and 12th electron.
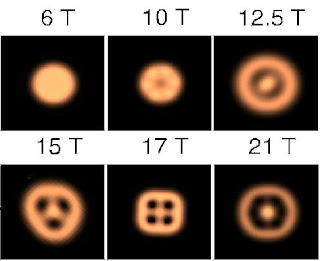
Quantum dots are 2D analogies for real atoms. But since they have much larger dimensions they are suitable for experiments that can not be carried out in atomic physics. It is especially interesting to observe the effect of a magnetic fieldd, B, on the atom-like properties. A magnetic flux-quantum in an atom requires typically a B-field as high as 10^6 T, whereas for dots this is of the order 1 T, which is experimentally accessible.
http://qt.tn.tudelft.nl/grkouwen/qdotsite.html this website is under contruction and quite interesting to read have a look at it ....
No comments:
Post a Comment