Monday, March 17, 2008
Monday, March 3, 2008
info on quantum dots continued
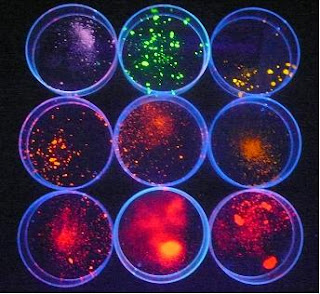 I know what you’re thinking-“Rich, what’s with all this about toxicity and ee-king? We can’t have our imaging material causing more problems.” And, you’re right. The scientists have also thought of this and have done experiments mirroring the body’s environment to make sure quantum dots and their coatings are stable over a broad range of PH and salt conditions- Even hydrochloric acid. And they passed with (ahem) flying colors.
I know what you’re thinking-“Rich, what’s with all this about toxicity and ee-king? We can’t have our imaging material causing more problems.” And, you’re right. The scientists have also thought of this and have done experiments mirroring the body’s environment to make sure quantum dots and their coatings are stable over a broad range of PH and salt conditions- Even hydrochloric acid. And they passed with (ahem) flying colors.Carbon nanotubes also have this fluorescence quality. R.Bruce Welsman and his group at
Down the road ,such carbon nanotube technology may be used along the same lines as the quantum dots.- you could end up wrapping the tubes with a specific protein allowing them to target cells (such as tumors) Along these lines a proposal by Michael Stranto and his team at the University of Illinois Urbana/Champaign(involving a glucose-detection optical sensor) looks especially promising. Here nanotubes are wrapped in a glucose oxidase and placed inside a small porous capillary (20 microns across by 1cm in length) the capillary pores are only big enough for glucose to penetrate. Once through the glucose promptly reacts with the oxidation solution changing the fluorescence properties of the nanotubes. This capillary is subsequently inserted just underneath the skin, but within range of being able to detect the near –infrared fluorescence, Imagine a patient with diabetes wearing a watch that periodically checks the fluorescence /glucose and sounds an alert if levels are too low or high- all without needles.
Unlike quantum dots, nanotubes don’t contain heavy metals, so they don’t raise any toxic issues. Additionally nanotubes can be fine-tuned to very narrow wavelength, providing fluorescence in a greater number of wavelengths, giving us greater flexibility( in other words nano colors to our palette) Such properties may give nano-tubes the advantage among products marketed as laboratory imaging markers.
More info on quantum Dots
 We describe quantum dots, semi conducting nanocrystals roughly 5nm in size. They also have applications in the biological world as fluorescent tags Quantum dots are nanometer-scale nanocrystals composed of a few hundred to a few thousand semiconductor atoms made out of bio-inert materials – meaning they are non intrusive and non toxic to the body additionally unlike fluorescent dyes (which tend to decompose and lose their ability to fluorescence), quantum dots maintain their integrity withstanding more cycles of excitation and light emission before they start to fade. Changing their size or composition allows scientist to cater their compositions allows the scientist to cater the optical properties.-Which means they can fluoresce in a multitude of colors. This effect is called quantum confinement (hence the name the quantum dots)- they have quantized discrete energy levels that are directly related to their size.
We describe quantum dots, semi conducting nanocrystals roughly 5nm in size. They also have applications in the biological world as fluorescent tags Quantum dots are nanometer-scale nanocrystals composed of a few hundred to a few thousand semiconductor atoms made out of bio-inert materials – meaning they are non intrusive and non toxic to the body additionally unlike fluorescent dyes (which tend to decompose and lose their ability to fluorescence), quantum dots maintain their integrity withstanding more cycles of excitation and light emission before they start to fade. Changing their size or composition allows scientist to cater their compositions allows the scientist to cater the optical properties.-Which means they can fluoresce in a multitude of colors. This effect is called quantum confinement (hence the name the quantum dots)- they have quantized discrete energy levels that are directly related to their size.Interestingly enough, quantum dots can even be tuned to fluorescence in different colors with the same wavelength of light. In other words it can choose quantum dots sizes where the frequency of light to make one group of dots fluoresce is an even multiple of the frequency required to make another group of dots fluoresce: both dots then fluoresce with the same wavelength of light. This allows for multiple tags to be tracked while using a singe light source.
A paul alivistos and his company (Quantum Dots corporation) have used these concepts in their Qdot product- a quantum dot surrounded by an inorganic shell that amplifies its optical properties while protecting the dot from its environment. The Qdot can have a variety of attachments to its shell. allowing it to attach to specific cell walls.- or even penetrate a cell and light it up from the inside. In the summer of 2003, This company joined forces with matsustha Electronic Industrial co(Panasonic) and sumitomo Corporation Biosciences to develop advanced optical and image processing technologies that utilize the Qdot. Products under this agreement are expected to generate revenue of more than $100 million per year for quantum dot Corporation by 2007.(Tiny product, big bucks)
An example of quantum dots in action involves targeting and imaging cancer cells. Researchers at
Putting Quantum dots to work
 Quantum dots apart from being neat things in and of themselves-could be put to some very interesting uses. The first application of quantum dots will be for biological labels used in medical imaging. Researchers tag proteins and nucleic acids with quantum dots. When they shine ultraviolet ray on the sample, the quantum dots glow at a specific wavelength and indicate the locations of attached proteins. Quantum dots have advantages over materials currently used for this application. For example they glow longer.
Quantum dots apart from being neat things in and of themselves-could be put to some very interesting uses. The first application of quantum dots will be for biological labels used in medical imaging. Researchers tag proteins and nucleic acids with quantum dots. When they shine ultraviolet ray on the sample, the quantum dots glow at a specific wavelength and indicate the locations of attached proteins. Quantum dots have advantages over materials currently used for this application. For example they glow longer.Researchers are also hoping that quantum dots could eventually provide energy efficient lighting for general use, in your house, office or neighborhood street lamps. In these applications a light emitting diode (LED) or other source of UV light would shine on quantum dots, which would then light up. By mixing different sizes (and the associated colors) of quantum dots together, you could generate white light. Generating light from quantum dots would work like generating fluorescent light but without the bulky fluorescent tube. This method would also avoid the wasted heat that you get with your typical incandescent light bulb.
Passing an electrical current through an LED also generates light. A company called Q vision is attempting to use techniques developed MIT to design a quantum dot LED: A layer of quantum dots sandwiched between conductive organic layers. Passing a c
Flat panel TV displays using quantum dots LEDs may provide more vibrant colors than current flat panel displays based on liquid crystals Display (LCD) Technology.
urrent through the dots generates light
Getting quantum dot energized

 Quantum dots are useful because when you add energy to their electrons, the electrons act they’re in one big atom.- and (as any physicist could tell you) When you add electrons in any atom, what you get is light. This occurs hen an electron moves to a higher energy level and then falls back again to it’s normal energy level. The same is true for quantum dots – zap them they will glow. One way to add energy to quantum dots is to shine an ultraviolet light on them
Quantum dots are useful because when you add energy to their electrons, the electrons act they’re in one big atom.- and (as any physicist could tell you) When you add electrons in any atom, what you get is light. This occurs hen an electron moves to a higher energy level and then falls back again to it’s normal energy level. The same is true for quantum dots – zap them they will glow. One way to add energy to quantum dots is to shine an ultraviolet light on themIt turns that the smaller the quantum dot, larger the gap between energy levels. Which means more energy is packed into photons – which means more energy is packed into the photon that’s emitted when an electron falls from a higher energy level to it’s normal energy level.A small quantum dot emits higher energy photons – with a shorter wavelength than a large Q-dot can.
So where do you get quantum dots (No you cant find them in one stop shops store at least not yet) It turns out that it is possible to grow a large number of quantum dots in a chemical reactions. But the methods used range from simple wet –chemical setups. (In which you precipitate zinc sulphide crystals) to complicate methods such as chemical-vapor deposition.(Which is also used to grow carbon Nanotubes). You can control the size of the particular batch of quantum dots- ensuring that they all emit the same wavelength of light- by controlling the length of time you allow the reaction to run. But what do you do with them once you have got them?
Making quantum leaps with quantum dots
Quantum dots are nano-size crystals that emit light; the wavelength they emit depends on the size of the crystal.
Quantum dots are composed of various materials such as lead sulfide, Zinc sulfide, cadmium selenide, and indium phosphide.
Quantum dots are useful because, depending on their size and composition they emit particular wavelength or color of light after an outside source such as the ultra violet light, excites the electron in them. Quantum dot produces light in a way similar to atoms. The ability to tailor the color of light emitted by a group of quantum dots is very useful in medical diagnostics
The rules that describe electron orbital (also called energy levels) and dictate that electrons are only allowed to be in certain energy levels within an atom are called quantum mechanics. Because electrons in this nano size crystals behave in a similar way they are called quantum dots.
Artificial atoms
 The three-dimensional (3D) spherically symmetric potential around atoms yields degeneracies known as shells, 1s, 2s, 3s, 3p,... Each shell can hold a specific number of electrons. The electronic configuration is particularly stable when these shells are completely filled wih electrons, occurring at 'magic' atomic numbers 2, 10, 18, 36,... In a similar way, the symmetry of a two-dimensional (2D), disk-shaped quantum dot leads to a shell structure with magic numbers 2, 6, 12, 20,... The lower degree of symmetry in 2D results in a different sequence of magic numbers than in 3D.
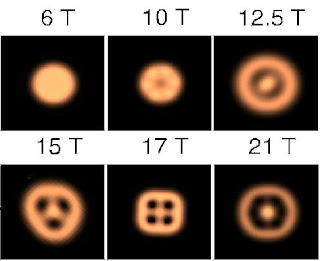
The three-dimensional (3D) spherically symmetric potential around atoms yields degeneracies known as shells, 1s, 2s, 3s, 3p,... Each shell can hold a specific number of electrons. The electronic configuration is particularly stable when these shells are completely filled wih electrons, occurring at 'magic' atomic numbers 2, 10, 18, 36,... In a similar way, the symmetry of a two-dimensional (2D), disk-shaped quantum dot leads to a shell structure with magic numbers 2, 6, 12, 20,... The lower degree of symmetry in 2D results in a different sequence of magic numbers than in 3D.By measuring electron transport through quantum dots, a periodic table of artificial 2D elements can be obtained. For this purpose, dots are connected via potential barriers to source and drain contacts. If the barriers are thick enough , the number of electrons on the dot, N, is a well defined integer. This number changes when electrons tunnel to and from the dot. However, due to Coulomb repulsion between electrons, the energy of a dot containing N+1 electrons is larger than when it contains N electrons. Extra energy is therefore needed to add an electron to the dot. Consequently, no current can flow which is known as the Coulomb blockade.
The blockade can be lifted by means of a third electrode closeby, known as the gate contact. A negative voltage applied to this gate is used to supply the extra energy and thereby change the number of free electrons on the dot. This makes it possible to record the current flow between source and drain as the number of electrons on the dot, and hence its energy, is varied. The Coulomb blockade leads to a series of sharp peaks in the measured current (see figure below). At any given peak, the number of electrons on the dot alternates between N and N+ 1. Between the peaks, the current is zero and N remains constant. The distance between consecutive peaks is proportional to the so-called addition energy, which is the difference in energy between dots with N+1 and N electrons. The magic numbers can be identified because significantly higher voltages are needed to add the 2nd, 6th and 12th electron.
Quantum dots are 2D analogies for real atoms. But since they have much larger dimensions they are suitable for experiments that can not be carried out in atomic physics. It is especially interesting to observe the effect of a magnetic fieldd, B, on the atom-like properties. A magnetic flux-quantum in an atom requires typically a B-field as high as 10^6 T, whereas for dots this is of the order 1 T, which is experimentally accessible.
http://qt.tn.tudelft.nl/grkouwen/qdotsite.html this website is under contruction and quite interesting to read have a look at it ....